New paper in Drug Delivery and Translational Research!
The article “Biosafety evaluation of etoposide lipid nanomedicines in C. elegans” has been published in Drug Delivery and Translational Research journal.
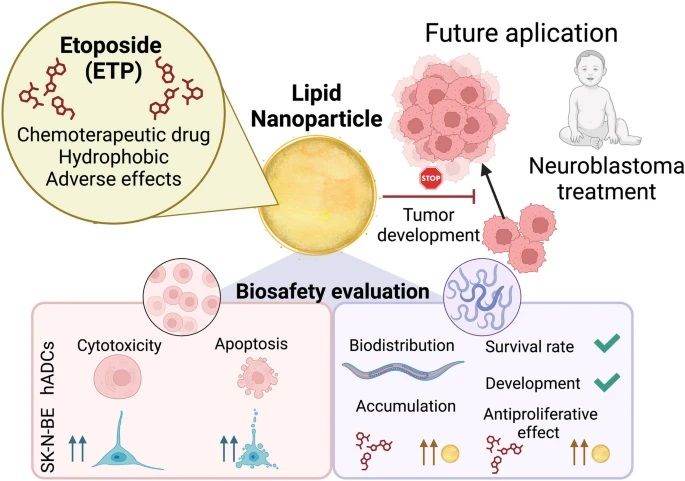
In this project, NN group members Amanda Muñoz and Anna Laromaine have collaborated with the group of María J. Blanco-Prieto (Universidad de Navarra and IdiSNA) for the evaluation of lipid nanoparticles with encapsulated Etoposide. They first evaluated the toxicity of empty and loaded nanoparticles in cancerous and healthy cell lines in vitro. The results demonstrated that etoposide nanomedicines exhibited high toxicity and selectively induced apoptosis only in cancerous cells. Next, the biosafety of these nanomedicines was evaluated in C. elegans by measuring survival, body size, and the effect on dividing cells. The findings showed that the nanomedicines had a safer profile than the free etoposide in this model. Notably, nanomedicines exerted etoposide’s antiproliferative effect only in highly proliferative germline cells. Therefore, the developed nanomedicines hold promise as safe drug delivery systems for etoposide, potentially leading to an improved therapeutic index for neuroblastoma treatment.
Abstract:
Neuroblastoma is a pediatric tumor that originates during embryonic development and progresses into aggressive tumors, primarily affecting children under two years old. Many patients are diagnosed as high-risk and undergo chemotherapy, often leading to short- and long-term toxicities. Nanomedicine offers a promising solution to enhance drug efficacy and improve physical properties. In this study, lipid-based nanomedicines were developed with an average size of 140 nm, achieving a high encapsulation efficiency of over 90% for the anticancer drug etoposide. Then, cytotoxicity and apoptosis-inducing effects of these etoposide nanomedicines were assessed in vitro using human cell lines, both cancerous and non-cancerous. The results demonstrated that etoposide nanomedicines exhibited high toxicity and selectively induced apoptosis only in cancerous cells.
Next, the biosafety of these nanomedicines in C. elegans, a model organism, was evaluated by measuring survival, body size, and the effect on dividing cells. The findings showed that the nanomedicines had a safer profile than the free etoposide in this model. Notably, nanomedicines exerted etoposide’s antiproliferative effect only in highly proliferative germline cells. Therefore, the developed nanomedicines hold promise as safe drug delivery systems for etoposide, potentially leading to an improved therapeutic index for neuroblastoma treatment.
Amanda Muñoz, Anna Laromaine, C. elegans, drug-delivery, Neuroblastoma

